Overview of Genetics
Our bodies are made up of cells that contain chromosomes. We have 23 pairs of chromosomes (46 in total), with one copy of each pair inherited from each of our parents. Our chromosomes contain our genetic material or DNA. Our DNA consists of segments called genes which provide the instructions, or the code, to create proteins that make us who we are. We all have variations in our DNA.
Sometimes variation occurs in our DNA that disrupts the code for making a protein. When this happens, the variation can cause a condition to develop, such as congenital hyperinsulinism (HI). These types of changes in the DNA are called disease-causing variants and have been found in over 30 different genes in individuals with HI. The genetic subtype of HI (which refers to the specific gene which is affected by a disease-causing variant) will determine whether HI occurs in isolation or is part of a syndrome. Recent studies have shown that a genetic diagnosis is possible in 50-70% of individuals with HI following routine testing.
You can access information about our Open Hyperinsulinism Genes Project – a collaboration with the University of Exeter to provide free genetic testing globally – here.
HI Genetic Subtypes
- K-ATP Channel (ABCC8 and KCNJ11): Inactivating variants in the ABCC8 and KCNJ11 genes that code for the pancreatic beta-cell ATP-sensitive potassium (K-ATP) channel. These channels act as a switch to ensure that insulin is only released under the proper conditions. When a disease-causing variant occurs in one of these genes, the channels do not function properly and insulin release becomes dysregulated, causing hypoglycemia. These variants cause diffuse pancreatic disease when dominantly or recessively inherited or focal disease when the variant is inherited from an unaffected father and there is concurrent loss of the mother’s chromosome 11 (loss-of-heterozygosity) within the pancreatic tissue. Further information on loss-of-heterozygosity and the different inheritance patterns are provided below. You can find more information in this article: Update of variants identified in the pancreatic β‐cell KATP channel genes KCNJ11 and ABCC8 in individuals with congenital hyperinsulinism and diabetes.
- Glutamate Dehydrogenase (GLUD1): Activating variants in the GLUD1 gene that code for the mitochondrial enzyme glutamate dehydrogenase (GDH). In this form of HI excess insulin secretion causes low blood glucose with fasting or when an individual eats protein. This type of HI is sometimes referred to as hyperinsulinism-hyperammonemia syndrome (HI/HA) as it causes high ammonia levels and, in some, people, may cause seizures. This condition can be dominantly inherited or can result from a variant that has occurred spontaneously (de novo) in the child during fetal development.
- Glucokinase (GCK): Activating variants in the GCK gene which codes for the beta-cell enzyme, glucokinase. GCK is the “glucose sensor” for the beta-cell. It tells the beta-cell how high the blood glucose is and when to secrete insulin. Variants in the GCK gene that instruct the beta-cell to secrete insulin at lower blood glucose than is normal cause HI. The age of diagnosis of HI due to an activating GCK variant ranges from birth to adulthood with varying severity of disease (some individuals may not have recognizable symptoms of HI). These variants can be inherited from a parent (dominant inheritance) or arise spontaneously (de novo).
- Hepatocyte Nuclear Factor 4 Alpha (HNF4A): Inactivating variants in the HNF4A gene which codes for a signaling protein (transcription factor). HNF4A regulates other genes involved with the development and function of pancreatic beta cells which are critical for regulating insulin secretion. The severity of disease ranges from mild transient HI (lasting a few days or weeks) to persistent HI which can continue into early- to mid-childhood. Individuals with HNF4A-HI are at increased risk of developing diabetes and will often have a parent or grandparent who is affected with early-onset diabetes. You can learn more about Mature Onset Diabetes of the Young (MODY) here.
- Hydroxyacyl-coenzyme A dehydrogenase (HADH): Inactivating variants in the HADH gene which codes for the mitochondrial enzyme Hydroxyacyl-coenzyme A dehydrogenase (sometimes referred to as Short-Chain 3-Hydroxyacyl-CoA Dehydrogenase [SCHAD]). The HADH gene controls insulin secretion by inhibiting the activation of glutamate dehydrogenase, an enzyme that has an important role in regulating amino acid-induced insulin secretion (see section above on GLUD1-HI). HADH-HI is a recessive form of HI that is often diagnosed between birth and late infancy.
- Other genetic subtypes and syndromic forms of HI: Over 20 further genetic subtypes of HI have been reported with new genes being discovered all the time. Other genes in which variants cause isolated HI include SLC16A1 which codes for the Monocarboxylate Transporter 1 (MCT1) and HK1 which codes for Hexokinase 1. Disease-causing variants in many genes are reported to cause syndromic HI where individuals have hyperinsulinism and additional clinical features. The most common forms of syndromic HI include Beckwith-Wiedemann Syndrome (BWS) due to anomalies on chromosome 11, Kabuki syndrome due inactivating variants in the KMT2D and KDM6A genes and Rubinstein-Taybi syndrome due to variants in the CREBBP and EP300 genes. For more information about these subtypes and other rare genetic causes of HI please visit: https://hyperinsulinismgenes.org/genetic-subtypes.
Basic Inheritance: Understanding inheritance patterns and your family history can help diagnose and determine the chance of developing a genetic subtype of HI.
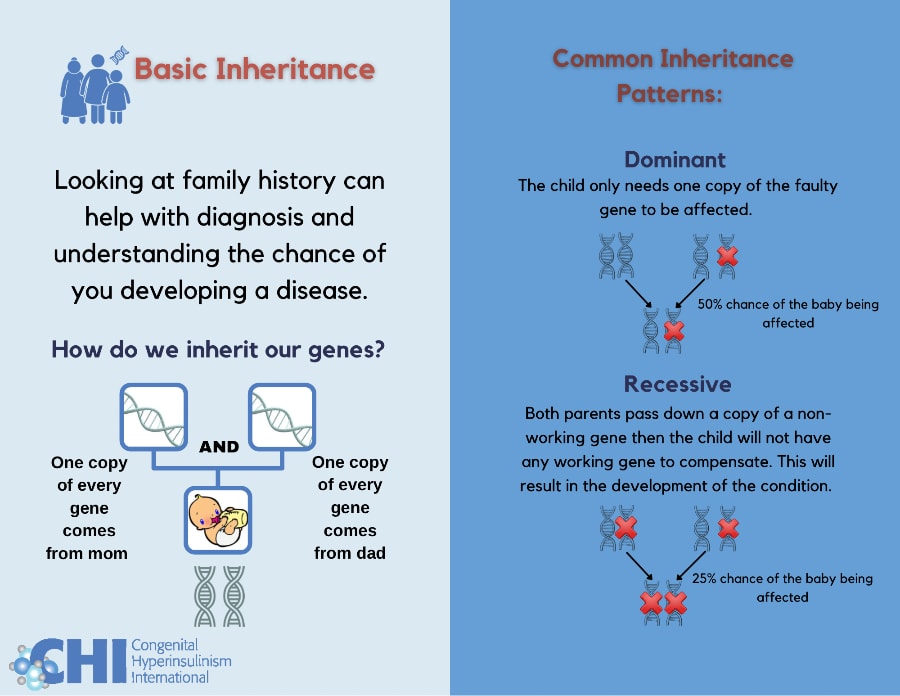
Types of Inheritance Patterns
Dominant: A child only needs one copy of the gene with a change to be affected. Dominant variants can arise spontaneously (known as a de novo variant) or be inherited from a parent who is also affected with the same condition. There is a 50% chance that a child born to an individual with a dominantly acting variant will inherit the variant and be affected with HI. Examples of dominantly inherited forms of HI include, GCK-HI, GLUD1-HI HK1-HI, HNF4A-HI and rare cases of ABCC8-HI.
Recessive: A child needs two copies of the gene with a change to be affected. The child will inherit one copy from their mother and one copy from their father who do not have HI but are ‘carriers’ of the condition. When both parents are a carrier of HI there is a 25% chance that a child will inherit both copies of the affected gene and develop HI. Examples of recessively inherited forms of HI include ABCC8-HI, KCNJ11-HI, and HADH-HI.
X-Linked: X-linked disease occurs when the gene with a change is on the X chromosome. Females have two copies of the X chromosomes, while males have one X and one Y Chromosome.
- X-linked recessive inheritance: To be affected with an X-linked recessive condition all copies of the X chromosome need to carry the affected gene. Females will therefore only be affected when both copies of their X chromosome contain the affected gene whilst males only need their one copy of the X chromosome to be affected.
- Dominant X-linked inheritance: Both females and males only need one copy of the affected gene which is present on the X chromosome to have the disease.
Examples of X-linked forms of HI include Simpson-Golabi-Behmel due to recessive variants in the GPC3 gene and Kabuki syndrome due to dominant variants in KDM6A.
Other useful genetic terms
Heterozygous: Refers to a change in DNA that affects one copy of a gene that has been inherited from a single parent.
Homozygous: Refers to two identical changes in DNA that affect both copies of a gene each inherited from a different parent.
Loss-of-Heterozygosity: A genetic event that occurs spontaneously during fetal development. In HI this refers to the process by which the mother’s copy of DNA that contains the ABCC8 and KCNJ11 genes is lost from a population of beta-cells within the pancreas. This causes focal HI only in cases where the child has also inherited a recessively acting ABCC8 or KCNJ11 variant from their unaffected father.
De novo variants: Sometimes variants occur spontaneously during fetal development or within the germ cells of the parents (sperm or oocytes (eggs)). The likelihood of parents having a second child affected with HI will depend on where the variant had arisen. In HI most de novo variants are dominantly acting. In these cases, there is a 50% chance that an individual with a de novo variant will pass on the same variant to their own children.
Mosaicism: Refers to different populations of cells in the body where some cells contain DNA that has a disease-causing variant and other cells do not. These mosaic variants arise spontaneously (de novo) during development and are not inherited from a parent. Mosaic variants are usually dominantly acting. An individual can pass on a variant to their own children if the variant is present in their germ cells (sperm or oocytes (eggs)).
Carrier: An individual who has a recessively acting variant affecting one gene who does not show any symptoms of the condition.
How Genetics May be Able to Predict HI Type (Diffuse or Focal)
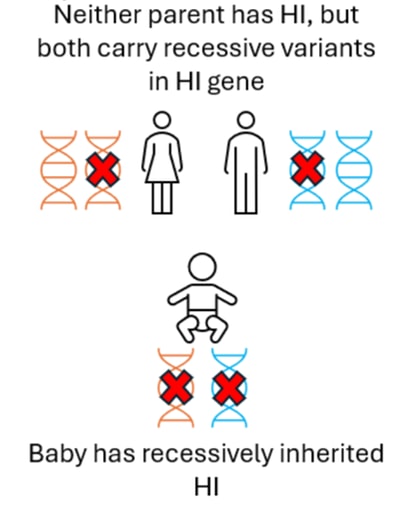
In some with Diffuse HI, an individual has two (recessive) variants in the ABCC8 or KCNJ11 gene. The child will have inherited one of these variants from their mother and the other from their father, neither parent will have HI.
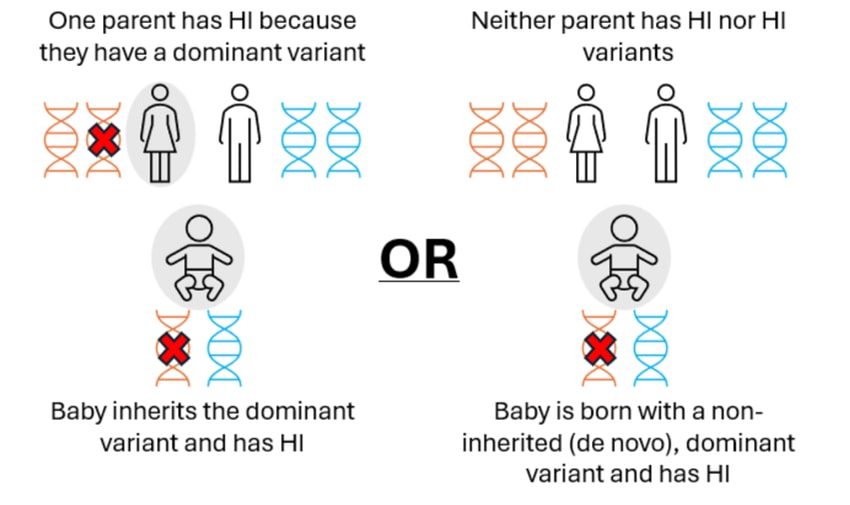
More rarely, diffuse disease can occur when the child has a single (dominant) variant in the ABCC8 or KCNJ11 gene. In these cases, the variant may have been inherited from a parent who also has HI or the variant may be new (de novo) in the child. These genetic variants can normally be detected within the blood sample.
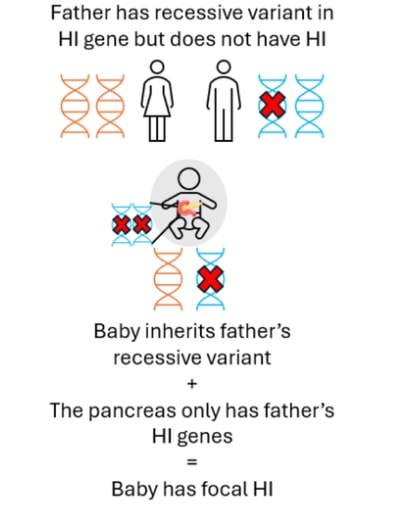
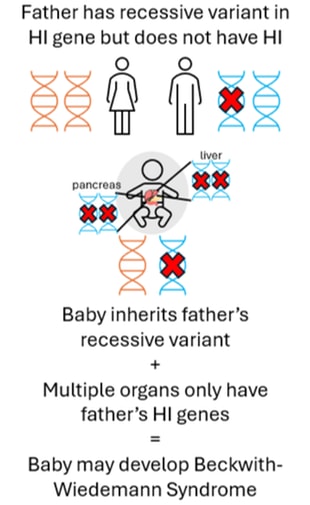
Focal HI arises after a two-step process. Firstly, a person inherits a single recessive genetic change in the ABCC8 or KCNJ11 genes from their father who does not have HI. This variant can usually be detected within the blood sample from the child and the father. Next, within the pancreas there is a loss of the ABCC8 and KCNJ11 genes which the child has inherited from their mother. This can only be detected by testing tissue from the pancreas, not the blood. Within the pancreas the ABCC8 and KCNJ11 genes are replaced by a copy of the father’s genes (a process referred to as paternal uniparental disomy (pUPD)). This means that within the child’s pancreas there are two copies of the ABCC8 and KCNJ11 genes from their father which has the HI variant and no copies of the ABCC8 or KCNJ11 genes from their mother. This causes the formation of a focal lesion within the pancreas. The rest of the pancreas is normal.
In some cases, the paternal uniparental disomy (pUPD – loss of a specific region of the mother’s DNA and subsequent gain of the father’s DNA) can occur in multiple tissues (i.e. it is not isolated to the pancreas. The image below shows the pancreas and liver affected but other tissues such as the kidneys may also be affected in the same way). When this happens the child may develop Beckwith-Wiedemann Syndrome spectrum (BWSp). If the child also inherits a genetic variant in ABCC8 or KCNJ11 from their father, then they may develop severe HI where diazoxide treatment is not effective.
Determining if You Have a Genetic Variation for HI
If HI is suspected, a primary care physician or endocrinologist may order genetic testing to understand the genetic cause and help determine treatment plans. Genetic testing is typically done using a saliva or blood sample, with the latter being the preferred option. This is because blood samples contain a higher amount of quality DNA required for testing by scientists.
Genetic Testing
Genetic testing is a diagnostic tool for identifying variation in your DNA that may be causing HI. Genetic testing can:
- help accurately diagnose conditions
- confirm the genetic cause of an already diagnosed condition
- help guide and stay proactive with treatment plans based on your personal genetics
- identify gene changes that can be passed down to children
Geneticists and genetic counselors will guide you on what genetic test is right for you and how to interpret your results.
Types of Genetic Tests
- Carrier Testing: This test is performed on prospective parents before pregnancy. If one parent has a gene for the disorder but does not have the condition, they are called a carrier. This gene can then be passed down to the baby, increasing the chances of the child having the condition. Genetic carrier testing can identify whether you and your partner are carriers of HI and the chances of your baby developing a genetic condition.
- Prenatal Testing: This type of testing is performed during pregnancy.
- Screening Test: These tests estimate the chances of your child having a birth defect associated with many different genetic disorders. These tests include blood tests and a specific type of ultrasound. Although screening tests cannot provide a definite diagnosis, they can indicate an increased risk for a genetic disorder, prompting further diagnostic options to be explored.
- Diagnostic Test: These tests can provide a definitive diagnosis of a genetic condition in an unborn child. Often these tests are invasive, such as chorionic villus sampling and amniocentesis. More recently a non-invasive prenatal testing has become available which includes screening of cell-free DNA (testing of mother’s blood for fetal DNA). This testing is only provided by highly specialized laboratories. Speak with your provider to understand your risks and benefits better.
- Postnatal or Predictive Test: This type of testing is performed after the baby is born, usually through blood or saliva collection to determine if the child has a genetic condition.
Results
There are three possibilities for genetic testing results:
- Positive Result: The laboratory has found a disease-causing variant in one of the genes known to cause HI. This type of result provides evidence for the cause of the HI and can help with treatment plans. Your physician or genetic counselor will go over these results with you.
- Negative Result: The laboratory did not find a disease-causing variant in any of the genes it was designated to test for. As more research is done, there is a possibility that new genes will be discovered that may be the reason for your HI condition. A negative result does not mean that you do not have HI; it just means that there is currently no genetic explanation for your diagnosis.
- Variant of Uncertain Significance (VUS): The laboratory has found a variant in the genes it was designated to test for; however, this variant has not previously been associated with HI. As more research is done, this VUS may be better understood and possibly reclassified as either benign (not clinically important) or disease-causing.
Please consult with a Geneticist and/or a genetic counselor for any specific questions. These professionals will be able to help guide you in interpreting and understanding your personalized results.
Cost
The cost of genetic testing can vary depending on the type of tests ordered, the laboratory, and insurance or healthcare plan. Please check your insurance or healthcare plan for more information.
In 2018 the team at Exeter joined forces with Congenital Hyperinsulinism International to provide funding for genetic testing to individuals who may otherwise be unable to receive screening. For details regarding this collaboration visit: https://congenitalhi.org/ and https://hyperinsulinismgenes.org
For individuals in the United States with HI who have received negative results from tier 1 and tier 2 genetic testing, the Broad Institute provides whole genome sequencing at no cost to families. For details regarding this project visit: https://cmg.broadinstitute.org
Turn Around
Time varies depending on which type of test and laboratory is chosen. However, early diagnosis can improve health outcomes. Talk to your provider for more information about turnaround times.
Process
If you go to a HI Center of Excellence for genetic testing, your sample will likely be sent to a laboratory that will test the ABCC8 and KCNJ11 genes first (tier 1). These tests often have a rapid turnaround and can help identify if a patient is likely to respond to diazoxide. If no disease-causing variant is found, they will move onto the second level (tier 2), testing other less commonly associated genes for HI. Specific genes can be tested if there is a family history of any genetic subtype of HI.
If you are in the U.S. and do not go to a Center of Excellence, your genetic test will likely be conducted by a commercial laboratory, which may test fewer genes.
If you are abroad and do not go to a Center of Excellence, depending on your level of access, you may qualify to have your genetic test completed at Exeter at no cost.
There are some benefits to having HI genetic tests sent to a laboratory that commonly works with HI genetics including:
- These labs test for a multitude of HI genes that may not be tested for at every site.
- Experts at these laboratories have extensive experience interpreting HI genetic tests.
- Your genetic changes can be checked against other individuals in a database to understand which variations may lead to HI. Since these labs tend to receive a majority of HI samples, they can better analyze your results.

