The Open Hyperinsulinism Genes Project: A Collaboration between Congenital Hyperinsulinism International, the Hyperinsulinism Research department and the Centre for Molecular Genetics at the University of Exeter (UoE) Medical School and Royal Devon and Exeter Hospital, Exeter, UK
Written in partnership with the University of Exeter Genomics Laboratory
Congenital Hyperinsulinism is a life-threatening condition where too much insulin causes dangerously low blood sugar. Genetic testing is required to guide medical management. Sadly, babies in resource-poor countries cannot access this.
To address these inequities, Congenital Hyperinsulinism International (CHI) teamed up with the University of Exeter Genomics Laboratory and the NHS National Hyperinsulinism genetics laboratory to establish a charity-funded testing service for those in need. Individuals are also invited to enroll in research to improve knowledge of the genetics and natural history of hyperinsulinism. Through this initiative we have turned constraints of limited resources into an advantage for scientific progress while improving the health of children worldwide. You can find additional details including contact and testing information here.
By bringing together a triumvirate of partners with the shared goal of equitable healthcare for congenital hyperinsulinism, we established the first point-of-need international genetic testing service for this condition and in doing so accelerated at scale scientific knowledge through the creation of a self-sustaining, research gene discovery pipeline.
The patient cohort established through this initiative represents the world’s largest data resource for congenital hyperinsulinism research. This is critically important as it will ensure that all new genetic discoveries will directly benefit their own populations and genetic knowledge is expanded by including previously unrepresented regions.
This initiative’s success has relied on the unique and complementary expertise of each partner, with CHI ensuring that the needs of the patient and patient family are placed firmly at the center of the project.
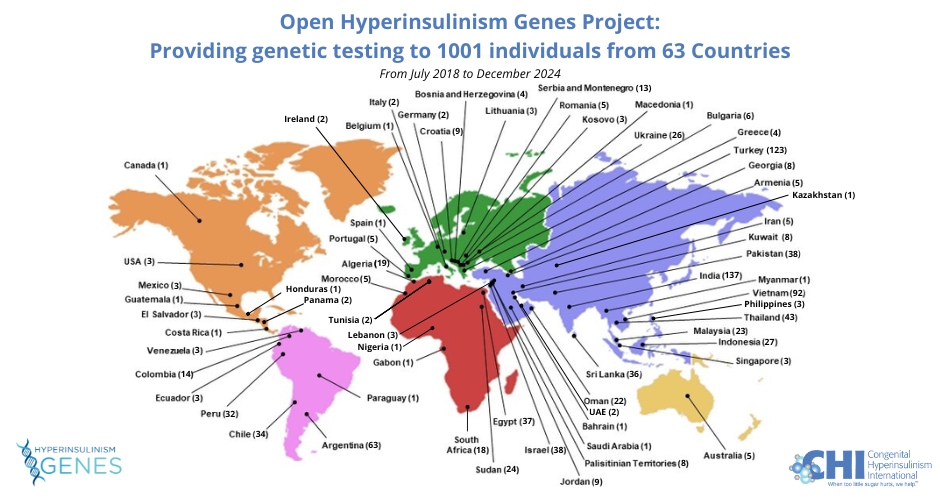
To date, we have enabled 1001 children born in resource-limited countries access to comprehensive testing for 21 of the known causes of hyperinsulinism. For every individual a full diagnostic genetic report has been issued, accompanied by an information sheet inviting families to join the Facebook online CHI Family Support Forum (to be included in the international community of patient families) and the HI Global Registry; a patient-driven research study (https://congenitalhi.org/higlobalregistry/).
523 / 1001 (52%) children have received a genetic diagnosis. This has guided medical management by informing on treatment decisions, prognosis, and recurrence risk of disease within families. Most notably for children with medically unresponsive disease, whose only treatment option is for their pancreas to be removed, finding the genetic cause determines if the whole organ needs to be resected leading to insulin-dependent diabetes, or whether a small lesion can be excised which is curative.
Full informed consent for research has been obtained from all those without a genetic diagnosis despite comprehensive testing and would like to participate. These families have all been recruited to Exeter’s Genetic Beta-Cell Research Bank (https://www.diabetesgenes.org/current-research/genetic-beta-cell-research-bank). Genome sequencing has been performed by the UoE team on a subset of individuals revealing three novel causes of disease to date (unpublished data, and Nature Genetics In Press). These research discoveries are quickly translated into clinical practice with all three genes now being routinely screened in all referrals to the NHS laboratory.
The outcomes of this partnership have been presented at multiple international meetings including the European Society of Pediatric Endocrinology (2021, 2022) and multiple CHI annual family conferences. This work has led to a collaboration with the Broad Institute, a Podcast hosted by Genuity Science, and a Facebook live streaming event for families.
IMPACT ON PATIENT CARE
Our partnership has enabled 967 individuals with congenital hyperinsulinism to receive comprehensive, genetic testing. These children were referred to Exeter from 63 countries across five continents.
The 2022 discovery by the UoE team that HK1 is a common cause (~5-10%) of congenital hyperinsulinism has been rapidly translated into routine diagnostics by the Exeter NHS team. This discovery bodes on previous work done by Dr. Charles Stanley at Children’s Hospital of Philadelphia (CHOP). CHI will be working with its partners to include HK1 in the first level of genetic testing at clinical laboratories around the world. The CHI Collaborative Research Network, funded by the Chan Zuckerberg Initiative Rare As One Project, has created a climate of sharing research discoveries at nascent stages, so patients receive the full benefits of research discoveries in a timely manner.
The critical role of this project for guiding medical management is gratefully acknowledged by the families and clinicians caring for these children:
“We want you to know that the contribution you make to us is invaluable. There is only gratitude towards you and your team.” Clinician, Argentina
“I would like to thank you for your laboratory’s kindness and promptness in helping us doing the genetic tests. The facilities available here are embryonic at best and the costs are prohibitive for poor families.” Clinician, India
“Thank you, we really appreciate your and the CHI Association’s kindness.” Mum, Israel
SCIENTIFIC IMPACT
In 2022, the UoE team discovered that variants in the HK1 gene cause congenital hyperinsulinism. By studying pancreatic tissue from patients, we confirmed that the changes in DNA cause an enzyme, which is normally absent in the pancreas, to be expressed in the organ leading to an over secretion of insulin and low blood sugar. This has led to new biological insights into beta-cell function and pathways of insulin secretion.
Excitingly, these results also provide a new mechanism for disease which will impact discovery efforts across the fields of endocrinology and human genetics. Importantly for families, this discovery provides a critical step towards identifying drug targets and realizing the goal of precision medicine for HK1-hyperinsulinism. The results of these studies have been written up for publication (Nature Genetics, In Press) and formed preliminary data for a Wellcome Trust Senior Researcher Fellowship awarded to Sarah Flanagan. This work is an exemplar of the scientific achievements brought about by this partnership.
In summary, through this partnership we have improved clinical outcomes and scientific knowledge of human disease, building on the ethos of equitable access to health without sacrificing quality or excellence. Our collaboration is a model for genetic discovery for other rare diseases.

