Download a PDF copy of the Frequently Asked Questions in the following languages, or scroll down for an interactive Q & A:
Helpful definitions related to registry participation
- HIGR – the shortened name for the HI Global Registry.
- Participant – the person diagnosed with or suspected of having congenital hyperinsulinism (HI).
- Caregiver – the parent or other authorized person who takes care of the Participant.
- Legally Authorized Representative (LAR) – the person who is legally authorized to provide consent to participate in research on behalf of someone who is unable to consent for themselves.
- Participant surveys – registry surveys that collect information about the Participant. Information may be entered by the Participants themselves if they are able to consent, or by their caregiver.
- Caregiver surveys – registry surveys that collect information about the Caregiver’s experiences caring for the Participant.
- Informed consent – this is a process in which you are informed about the purpose, risks, and benefits of joining this registry. You are free to decide whether to join the registry, and you can change your mind and withdraw your consent to participate at any time.
- De-identified – this is a process in which all information that may identify you, such as your name, date of birth, address, etc., are removed from the data. This step is taken to ensure your privacy.
- Aggregated – this is the process in which data from individual participants is combined to provide a summary of all participants, such as the average or the total number.
- Study Sponsor – the organization that is responsible for the initiation, management, and financing of the registry and making sure it meets appropriate regulations and standards. Congenital Hyperinsulinism International (CHI) is the sponsor for the HI Global Registry.
- Registry Steering Committee – a collection of international HI representatives that provide oversight and expertise to the design and operation of the registry from the patient, parent, and clinical professional perspectives [See: https://congenitalhi.org/hi-gr-steering-committee/].
- Institutional Review Board (IRB) – a diverse group of qualified individuals that have been formally designated to review research to assure the protection of the rights and welfare of research participants. To accomplish this, IRBs use a group process to review research protocols and related materials (e.g., informed consent documents and investigator brochures).
Understanding the HI Global Registry (HIGR)
What is a patient registry?
A patient registry is an organized way of collecting information about a group of individuals with the same or related diseases. The HI Global Registry contains a series of surveys designed to capture information about various aspects of being diagnosed with and living with hyperinsulinism.
Why should I join the registry?
You can contribute to HI research by joining the registry. Researchers can use data from the registry to understand the disease better, which may lead to advances in HI recognition, management, or new treatments.
Who is eligible to join the registry?
Anyone who:
- Has been diagnosed with HI
- Is suspected of having HI
- Previously had HI and is now cured
There is no minimum or maximum age to participate in the registry. Individuals with HI who are over the age of 18 and are able to consent can create their own registry account, and caregivers may create an account on behalf of their child or an adult who is unable to consent. Caregivers may also join the registry on behalf of an individual with HI who has passed away.
What are the benefits of joining the registry?
Rare diseases such as HI are difficult to study because there aren’t many people with the condition, and they are generally spread all over the world, making it challenging to collect for research or clinical trials. The HI Global Registry is available online, so you can participate and contribute to HI research from anywhere in the world.
Participating in the registry helps to build our knowledge of the disease, outcomes, and clinical management practices. Researchers can use data from the registry to better understand HI. Having an available registry of information about HI may help to speed up research, which could eventually help researchers learn whether or how treatments work, help medical professionals improve how they treat the disease, and can eventually lead to a cure.
Registry participants may receive information about opportunities to participate in other research or clinical trials, as well as information about medical advances and ways to connect with others in the HI community.
What are the risks of joining the registry?
There is minimal risk in taking part in the registry. You may feel uncomfortable answering some of the survey questions about your medical history, but you do not have to answer any questions that you do not want to answer. Like with any other internet-based activity, there is a possible but unlikely risk of a breach in the registry system or a risk that your data may be misused. We have protections in place to protect your privacy and lower the risk of a data breach.[:es]There is minimal risk in taking part in the registry. You may feel uncomfortable answering some of the survey questions about your medical history, but you do not have to answer any questions that you do not want to answer. Like with any other internet-based activity, there is a possible but unlikely risk of a breach in the registry system or a risk that your data may be misused. We have protections in place to protect your privacy and lower the risk of a data breach.
Can I participate from any country in the world?
Yes, we encourage everyone to participate from anywhere in the world.
What languages are the registry surveys available in?
All registry surveys are currently available in English. Spanish and German will be available in late 2023, and Italian, Portuguese, French, and Korean will be available in 2024. We hope to add additional languages in the future.
What information will the registry collect?
The registry surveys collect information on the participant's family history of HI and medical information such as symptoms, medications, glucose monitoring, surgical history, and genetic test results. There are also two quality-of-life surveys (one for Participants age 14+ years and one for Caregivers) that collect information about Participants' and Caregivers' feelings about life with HI.
The 13 base surveys in the registry are:
Survey Name: Contact and Demographics
Purpose: Collects your preferred method of contact
Survey Name: MaxHIGR
Purpose: To give permission (optional) for registry staff to contact the Participant's physician to complete a physician survey called MaxHIGR. MaxHIGR collects information relating to your HI from your medical records, such as lab values at the time of diagnosis, genetic test results, and details of any surgeries. This physician-generated information complements the patient-reported responses from HIGR.
Survey Name: Glucose Monitoring and Management
Purpose: Collects information on methods of glucose monitoring, barriers to accessing monitoring devices, and general stability of blood glucose.
Survey Name: Diagnosis
Purpose: Collects information on different aspects of the medical investigation leading to a diagnosis of HI, including family history, genetic testing, and imaging.
Survey Name: Other Medical Conditions
Purpose: Collects information on other medical conditions that may be associated with HI, such as epilepsy, diabetes, and pancreatic insufficiency.
Survey Name: Medication Management
Purpose: Collects information on past and present medications, side effects, and access issues.
Survey Name: Diet and Feeding
Purpose: Collects information on past and present methods of feeding and feeding issues.
Survey Name: Development
Purpose: Collects information on developmental milestones, complications, and therapies.
Survey Name: Surgical Management
Purpose: Collects information on pancreatic surgery and outcomes.
Survey Name: Pregnancy
Purpose: Collects information on the biological mother’s pregnancy and health and the Participant's health before birth.
Survey Name: Birth
Purpose: Collects information about the Participant's health during birth and in the first few days of life.
Survey Name: Quality of Life - Participant (14+ years)
Purpose: Collects information about the Participant's feelings on how HI impacts their life.
Survey Name: Quality of Life - Caregiver
Purpose: Collects information about the Caregiver’s feelings on how caring for someone impacts their life.
How will the information I provide to the registry be used?
Information collected in this registry will be de-identified, combined, and then used in medical research to document the natural history and outcomes of individuals with HI. This research may include studies to better understand HI, to develop new treatments, or to inform future clinical trials.
Only authorized HIGR staff will have access to any single individual’s information in the registry. Qualified researchers who sign a data use agreement (agreeing to protect your privacy and not make any attempts to try to identify you) may be granted access to de-identified data. Most requests will be for aggregate data, this is summary data from all registry participants, such as averages and totals.
What are my options if I do not want to be in the registry?
You do not have to join this registry. Your participation is voluntary. You do not need to participate in this registry to remain a member of the HI community. Your decision about whether or not to participate in this registry will not affect your healthcare, medical treatment or insurance benefits. If you join the registry and later decide you do not want to participate, you may withdraw your consent from the registry at any time, for any reason. You do not have to tell us the reason.
I joined HIGR and completed surveys when the registry was hosted on the NORD IAMRARE platform. What happens to my data now the registry has moved to Matrix?
All the survey information you entered previously has been securely transferred to the new Matrix platform. NORD will still be able to use the de-identified data for research studies but will not have any access to your contact information and will not receive any new data you enter into the registry.
Joining the registry
What do I need to do if I choose to join the registry?
- Create an account at higlobalregistry.org
- Provide consent to participate in the registry
- Add a Participant (if you are a caregiver joining on behalf of someone with HI)
- Complete the registry surveys
- Update your survey responses as needed or every 6 months for some surveys
How much time does it take to join the registry?
Creating an account, providing consent, and adding a Participant typically takes less than 15-20 minutes. Once you have set up your account, the dashboard will display all available surveys and provide an estimated completion time for each survey. Some surveys are very short and will only take 1-5 minutes to complete; other surveys are longer and may take up to 30 minutes to complete. We encourage you to complete all surveys, even if they may not seem relevant.
How much time does it take to complete the surveys?
Some surveys are very short and will only take 1-5 minutes to complete; other surveys are longer and may take up to 30 minutes to complete. Many surveys use branching logic and will only ask certain groups of questions based on your response to a previous question. This means that depending on responses throughout the survey, some surveys may be very short for some people and longer for others.
A good example of this is the Surgical Management survey. This survey is very short for Participants who report that they have not had a pancreatectomy, but a little longer for those who have had a pancreatectomy, simply because there are more questions asked related to the surgery.
You may complete surveys at your own pace. You do not have to complete all the surveys at once. You may save a survey as a draft and complete it later.
What do I do once I have completed all the surveys?
Thank you for your contributions to HI research! By completing all registry surveys, you help to paint a complete picture of your HI experience and ensure we are collecting information on all aspects of life with HI.
Make sure to keep us updated on anything that changes, such as changes to medications, additional surgeries, genetic test results, or newly diagnosed medical conditions. To update your survey responses, navigate to Completed Surveys and click on the three dots to the right of the survey that needs to be updated. Then select “Retake”. A new version of the survey will be generated, and your previous responses will be automatically populated. Simply update your responses and click “Complete” to submit your updated responses.
You will receive a Dashboard survey every six months, prompting you to think about anything that might have changed. If you indicate in the Dashboard survey that something has changed, you will be prompted to review the appropriate survey to update your responses.
The registry includes two Quality of Life surveys (one for Participants age 14+ years and one for Caregivers) and a Glucose Monitoring survey, which are scheduled to be taken every six months. It is important to continue to take these surveys every six months so researchers can learn about how your perspectives and feelings on life with HI and your glucose monitoring regimens change over time. Researchers may be able to draw conclusions about how aspects of managing HI, such as medications or glucose monitoring, affect your quality of life.
Will I need to provide any updates to the registry information?
Having accurate, up-to-date information on all registry participants ensures we provide the most accurate data for HI research. We ask that you update your registry surveys any time something significant changes, such as changes to medications, new pancreatic surgery, genetic test results, or new medical conditions.
You will receive a Dashboard survey every six months, prompting you to think about anything that might have changed. If you indicate in the Dashboard survey that something has changed, you will be prompted to review the appropriate survey to update your responses.
The registry includes two Quality of Life surveys (one for Participants age 14+ years and one for Caregivers) and a Glucose Monitoring survey, which are scheduled to be taken every six months. It is important to continue to take these surveys every six months so researchers can learn about how your perspectives and feelings on life with HI and your glucose monitoring regimens change over time. Researchers may be able to draw conclusions about how aspects of managing HI, such as medications or glucose monitoring, affect your quality of life.
Can I register on behalf of someone else, such as my child or spouse?
Yes, you can create an account for someone with HI who does not have the capacity to consent to research and manage their own registry account, such as children under the age of 18 and adults with neurological deficits who do not have the legal capacity to consent to research. You will answer participant surveys on behalf of the Participant with HI, and you will also be assigned your own caregiver surveys (such as the caregiver Quality of Life survey).
All adults over the age of 18 who have the legal capacity to consent to research must create their own registry account.
Can I register on behalf of someone who has passed away?
If you are the parent/guardian or legally authorized representative of someone with HI who has passed away, you may create an account and answer registry surveys on their behalf. When creating an account, there will be an option to indicate that you are a "Person who has lost a loved one."
Can I register if I used to have HI but am now cured?
Yes, we strongly encourage all people who are now cured and no longer living with HI to register. It is important to investigate the long-term outcomes after HI is cured, but people with resolved HI are currently underrepresented in HI research.
Can I register if I suspect I have HI but have not yet received a formal diagnosis?
Yes, anyone who has a strong clinical suspicion of hyperinsulinism is welcome to join HIGR. You do not need to have a genetic diagnosis in order to join.
Can I register if I have a HI-related condition, such as Beckwith-Wiedemann syndrome?
Yes, anyone with any HI-related condition is welcome to join HIGR.
Does my doctor need to be involved or give permission for me to join this registry?
No, your doctor does not need to give permission for you to join this registry. However, there is one survey, MaxHIGR, that can only be completed by your physician. MaxHIGR collects information relating to your HI from your medical records, such as lab values at the time of diagnosis, genetic test results, and details of any surgeries. You can choose whether or not to give us permission to contact your physician to complete this survey. You can still join HIGR and complete participant and caregiver surveys if you choose not to give us permission to contact your physician for MaxHIGR.
What if I change my mind after joining the registry?
You may withdraw your consent to participate in the registry at any time for any reason. You do not have to tell us the reason.
You can withdraw your consent by contacting support through the "Contact Us" menu item on the registry dashboard or by emailing MatrixSupport@AcrossMatrix.com.
If you choose to withdraw from the registry after completing one or more surveys, your data will be removed from the registry and will not be included in any future studies using registry data. Data that has already been used in a research study or shared with qualified researchers prior to your withdrawal cannot be retrieved or deleted.
I joined HIGR on behalf of the participant when they were a child. How do I transfer the registry account to the participant after they turn 18?
Contact MatrixSupport@AcrossMatrix.com to request this change. You will need to provide a valid email address for the participant.
Connecting a glucose monitoring device to HIGR to share glucose values
How do I connect my glucose monitoring device to HIGR?
- Log in to HIGR and view a patient’s dashboard (not the caregiver dashboard)
- Select "Apps and Devices" in the navigation pane on the left. If you don't see "Apps and Devices," click the person icon in the top right corner and switch to a patient dashboard.
- Click "Connect" on the card for your device, and follow the instructions to log in and give permission to share your glucose values with HIGR.
- How do you view your CGM values?
- Smart device (e.g. phone or watch): No extra steps required!
- Handheld receiver: Upload your CGM values to Dexcom Clarity once per month to send data to HIGR. Note: We encourage everyone with a compatible smart device to download the Dexcom app to avoid needing to manually connect the receiver to Dexcom Clarity.
- Any questions? See our PDF Guides for a step-by-step guide with screenshots, or get in touch at info@higlobalregistry.org
Which glucose monitoring devices are currently supported for data sharing via HIGR?
Please see here for the list of glucose monitoring devices that HIGR currently supports for sharing glucose monitoring data. We are actively working to enable data collection from more devices.
Unfortunately, Dexcom CGM data cannot be collected from users living in Singapore or Japan at this time.
My device is not currently supported for data sharing. Can I share my glucose monitoring data another way?
We are actively working to enable data collection from more glucose monitoring devices. To make sure your device is included in this list, please email info@higlobalregistry.org and let us know what device you use.
My HI has transitioned to diabetes. Can I still share my glucose monitoring data?
Yes! It is so important to learn more about all experiences with HI, including from those who have transitioned to diabetes. Please connect your glucose monitoring device(s) to share your data, and make sure your survey responses are up-to-date so we know that you have diabetes.
The most important surveys to update are:
- "Other Medical Conditions" - to report that you have been diagnosed with diabetes
- "Diagnosis" - to report if your HI has resolved
If you are unsure how to update surveys, please see our PDF Guide: How to Update HIGR Surveys or contact us with questions at info@hiblogablregistry.org
I use a Dexcom receiver to view my CGM values instead of a smart device. Can I still share my data with HIGR?
Yes, although individuals who use a Dexcom receiver need to manually upload their data to Clarity once per month to send data to HIGR. We can only collect a limited amount of historical data from your device, so you need to upload data once per month to ensure that no data is missing from HIGR.
We encourage everyone with a compatible smart device to download the Dexcom app to avoid needing to manually upload data to Clarity.
What will my glucose monitoring data be used for?
Like all data collected in HIGR, your glucose monitoring data will be studied by HI researchers to help them learn more about HI. Glucose data can be paired with your HIGR survey responses to help HI researchers understand how glucose management is impacted by age, HI type, treatment options, medications, etc. As with all information you provide to HIGR, your privacy is our top priority. Your data is always de-identified for research studies, which means your name, date of birth, and any other information that could identify you is removed before your data is shared with researchers.
Costs and Compensation
Will I be compensated for being in this registry?
No, you will not receive any financial compensation for being in this registry.
There may be additional research opportunities that may provide compensation, which you may be notified of if you indicated in your contact preferences that you would like to be notified of additional research opportunities. There is no guarantee that you will be contacted about additional compensated research studies.
Will it cost me anything to be in this registry?
No, it will not cost you anything to be in this registry. Neither you nor your insurance carrier will be charged for your participation in this registry.
Data Storage, Privacy, and Confidentiality
Who will have access to my information within the registry?
Authorized HIGR staff who have been trained in data privacy and security are the only people who will have access to your full registry information, including information that could identify you and your contact details. HIGR staff will only contact you with your consent and will never share your contact information with anyone.
Will my information be shared with anyone?
If you meet the requirements for a third-party study, HIGR research staff will provide you with basic information about the study and a way to contact the researcher if you wish to do so. The third-party researcher will not have any of your contact information and will not contact you directly.
De-identified may be shared with qualified researchers who have submitted an application to use HIGR data for HI research.
De-identified data may be shared with other databases, such as the Rare Disease Cures Accelerator-Data and Analytics Platform (RDCA-DAP®), the National Organization for Rare Disorders (NORD), and the Global Rare Disease Patient Registry Data Repository (GRDR). This will allow more researchers to use the de-identified information for rare disease research.
What steps are taken to protect my privacy?
HIGR staff are committed to doing everything within their power to protect your privacy when collecting and storing your medical information. Only HIGR research staff have access to the information that could identify you, and HIGR staff will only contact you according to your preferred method of contact with your consent.
Qualified researchers who apply to use HIGR data in HI research only have access to de-identified data. Researchers are required to sign a Data Use Agreement, agreeing to take all necessary steps to continue to protect your privacy and not misuse data before gaining access to HIGR data.
Although we will take every reasonable measure to protect your privacy and confidentiality, because HI is rare, there is a small risk that you may be identifiable from the information in the registry.
What is de-identified data? What is aggregated data?
De-identified data is data that has had all of the information that may identify you, such as your name, date of birth, address, etc., removed. Aggregated data is data that summarizes the data across all individuals without sharing each individual participant's data. These steps are taken to ensure your privacy.
Table 1 below shows an example of how personal identifiers are removed to create de-identified data.
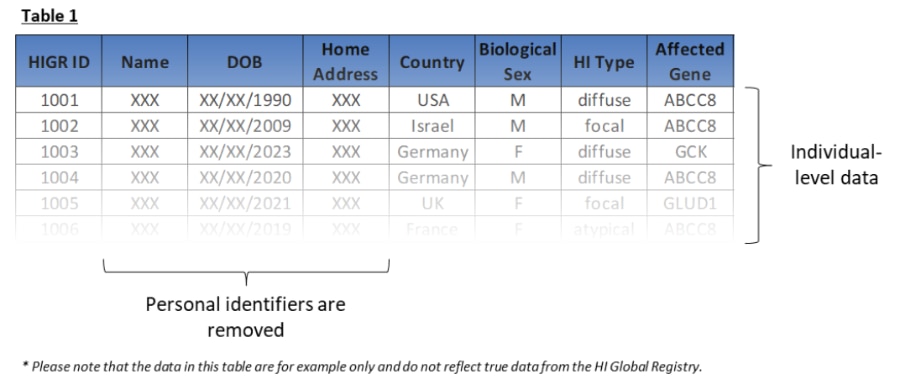
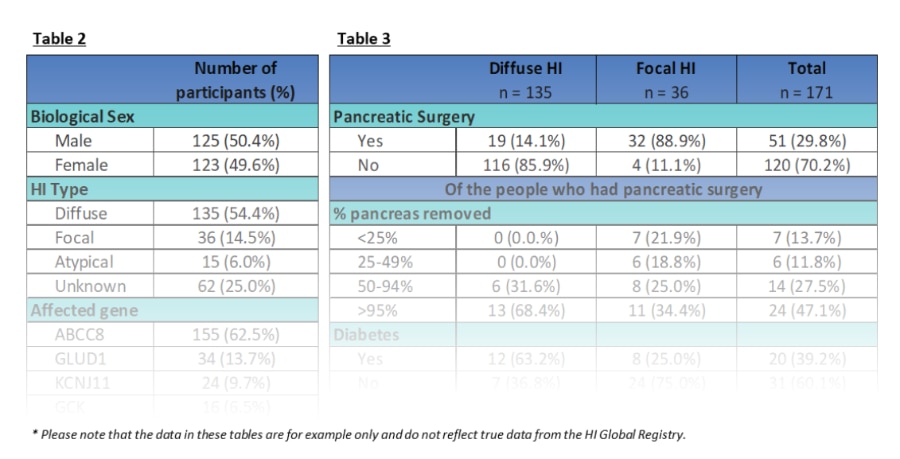
Tables 2 and 3 below show how de-identified data is aggregated to provide a summary of the data from all participants without sharing individual-level data. Most data requests from authorized researchers are for aggregate data.
Where is the registry hosted?
HIGR is hosted on the Matrix platform by Across Healthcare. You can learn more about Matrix at their website.
Who provides oversight for the registry?
HIGR is governed by the HIGR Steering Committee, which is a group of over 20 internationally recognized HI advocates, clinicians, and researchers representing 6 countries across the world. The Steering Committee was heavily involved in designing the registry surveys and continues to provide feedback and guidance on all registry-related activities. You can learn more about the members of the steering committee here.
In addition, the Institutional Review Board (IRB) reviews all registry-related documents, such as surveys, informed consent documents, investigator brochures, email templates, and promotional materials. The members of the IRB review these documents to ensure the protection of the rights and welfare of registry participants.
Who owns the registry data?
HIGR data is owned by Congenital Hyperinsulinism International (CHI), a leading nonprofit dedicated to improving the lives of children and adults living with HI. CHI, with input from the Steering Committee, decides which qualified researchers may be permitted to use de-identified data from HIGR in their research and which limited aggregated data may be shared with industry researchers to inform their research studies and clinical trials. CHI does not share your contact information with anyone.
Who funds the registry?
HIGR is funded by a combination of generous donations and sponsorship from industry partners. Congenital Hyperinsulinism International (CHI) staff manage the registry with input from an independent Steering Committee. Funding parties are not involved in the management of the registry or the design of registry surveys.
What happens to my data if I withdraw from the registry?
If you choose to withdraw from the registry after completing one or more surveys, your data will be removed from the registry and will not be included in any future studies using registry data. Data that has already been used in a research study or shared with qualified researchers prior to your withdrawal cannot be retrieved or deleted.
Clinical Trial Opportunities
Is HIGR a clinical trial?
No, HIGR is not a clinical trial. HIGR is a registry that collects information from individuals with HI and their caregivers about their health and other aspects of their lives with HI.
Will I be contacted about future research opportunities?
If you join the registry, you will have the opportunity to opt-in to being contacted if you potentially meet the requirements to participate in another research study. Other research studies may include sub-studies of HIGR, clinical trials, or other types of research studies conducted by third-parties. If you meet the requirements for a third-party study, HIGR research staff will provide you with basic information about the study and a way to contact the researcher if you wish to do so. The third-party researcher will not have any of your contact information and will not contact you directly.
There is no guarantee that an individual Participant will be eligible for a particular trial or contacted about a clinical trial. Even if you are contacted about possible eligibility based on information in the registry, you may or may not meet the study requirements.
I want to be involved in a clinical trial. If I register, is this guaranteed?
Although one of the main goals of the registry is to make it easier for Participants to take part in research, there is no guarantee that an individual Participant will be eligible for a particular trial or contacted about a clinical trial. Even if you are contacted about possible eligibility based on your information in the registry, you may or may not meet the study requirements.
Other Questions
Can I share my registry data with my healthcare provider?
You may request an email containing a PDF of your survey responses, which you can share with anyone, including your healthcare provider, if you choose to do so.
What kind of support or resources are available for participants?
HIGR staff are available to help you with any issues you may encounter when joining the registry or completing surveys.
- Email: info@higlobalregistry.org
- Phone: +1-973-842-7559
- See our Guides and Tutorials
- Join the CHI Facebook forum
You may also drop by one of our HIGR Virtual Office Hours or an in-person booth at a meeting or conference. Please see our Events page to find out where were going to be next!
Can I refer others to join the registry?
Yes - the more people who join HIGR, the stronger the data becomes! You may invite a friend to join HIGR by clicking the "Refer a Friend" menu item on your registry dashboard. Complete their name and email address, and we'll send them an invitation.
What happens if I forget my login credentials?
Contact the Matrix Support team at matrixsupport@acrossmatrix.com to reset your login details.
How can I contribute to improving the registry?
You can help to improve the registry data in multiple ways:
- Make sure all of your surveys have been completed and are up to date
- Spread the word and invite other people with HI and/or their caregivers to join HIGR
- If you have feedback on survey design, questions, or new survey topics you would like to see, email us at info@higlobalregistry.org
I have more questions
Who do I contact if I have more questions?
If you have questions about the registry, you can contact the HIGR staff at:
- Email: info@higlobalregistry.org
- Phone: +1-973-842-7559
If you have technical problems with the registry, you can contact Matrix at:
Who do I contact if I have a complaint?
If you have a complaint or feedback you would like to share with HIGR staff, you can contact us at:
- Email: info@higlobalregistry.org
- Phone: +1-973-842-7559
If you have concerns that you do not want to share with HIGR staff, you can contact the Institutional Review Board at:

